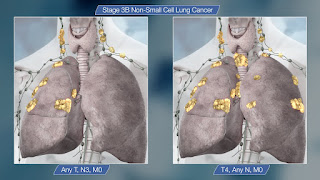
Non-Small Cell Lung Cancer (NSCLC) Clinical Presentation: History, Physical Examination

pulmonologist ut southwestern :: Article Creator FDA Approves Macitentan, Tadalafil Combination Tablet For Pulmonary Arterial Hypertension The approval, which marks the first for a once-daily, single-tablet combination therapy for pulmonary arterial hypertension, is based on findings from the phase 3 A DUE study. The FDA has approved a single-tablet combination of macitentan and tadalafil (Opsynvi; Johnson & Johnson) for the chronic treatment of pulmonary arterial hypertension (PAH) in adults who are treatment-naïve or are already on an endothelin receptor antagonist, phosphodiesterase 5 inhibitor, or both, according to a press release from Johnson & Johnson.1 The combination is indicated for patients with PAH categorized as WHO Group I and WHO functional class II-III, and the tablet can be used in those being treated with stable doses of macitentan 10 mg and tadalafil 40 mg (taken as two 20 mg tablets), as separate tablets. FDA ...